research use only
Resminostat HDAC inhibitor
Cat.No.S2693
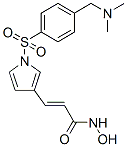
Chemical Structure
Molecular Weight: 349.4
Quality Control
Solubility
|
In vitro |
DMSO
: 70 mg/mL
(200.34 mM)
Ethanol : 70 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 349.4 | Formula | C16H19N3O4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 864814-88-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | RAS2410 | Smiles | CN(C)CC1=CC=C(C=C1)S(=O)(=O)N2C=CC(=C2)C=CC(=O)NO | ||
Mechanism of Action
| Targets/IC50/Ki |
HDAC1
42.5 nM
HDAC3
50.1 nM
HDAC6
71.8 nM
|
|---|---|
| In vitro |
Resminostat [HCl] is acting as a potent inhibitor of recombinant HDAC 1, 3 and 6 isoenzymes with a substrate competitive binding mode. It can induce hyperacetylation of histone H4 in MM cells. Low micromolar concentrations of this compound abrogates cell growth and strongly induces apoptosis in MM cell lines (OPM-2, NCI-H929, U266 ) as well as primary MM cells. At 1 μM, this chemical inhibits proliferation and induces G0/G1 cell cycle arrest in OPM-2, NCI-H929, U266 MM cell lines accompanied with decreased levels of cyclin D1, cdc25a, Cdk4 and pRb as well as upregulation of p21. This compound decreases phosphorylation of 4E-BP1 and p70S6k indicating an interference with Akt pathway signalling. Treatment with this agent results in increased protein levels of Bim and Bax and decreases levels of Bcl-xL. Caspases 3, 8 and 9 are activated by it. Furthermore, synergistic effects are observed for combinations of this compound with melphalan and the proteasome inhibitors bortezomib and S-2209.
|
| Kinase Assay |
Enzymatic HDAC activity assays
|
|
Forty microliter enzyme buffer (15 mM Tris HCl pH 8.1, 0.25 mM EDTA, 250 mM NaCl, 10% v:v glycerol) containing HDAC1, 3, 6 or 8 activity, 29 μL enzyme buffer and 1 μL resminostat [HCl] at different concentrations are added to a 96-well microtitre plate and the reaction started by the addition of 30μL substrate peptide Ac-NH-GGK(Ac)-AMC (HDAC1, 3 and 6 assays, final concentrations 6 μM for HDAC1, 10μM for HDAC6 and 25μM for HDAC3/DAD) or Ac-RHK(Ac)K(Ac)-AMC (HDAC8 assay, final concentration 50 μM). After incubation for 180 min (HDAC1, HDAC6, HDAC8) or 120 min (HDAC3) at 30°C, the reaction is terminated by the addition of 25 μL stop solution (50 mM Tris HCl pH 8, 100 mM NaCl, 0.5 mg/ml trypsin and 2 μM trichostatin A [TSA]). After incubation at room temperature for further 40 min, fluorescence is measured using a multilabel counter (extinction 355 nm, emission 460 nm) for quantification of AMC generated by tryptic cleavage of the deacetylated peptide. For the calculation of the 50% inhibitory concentration (IC50) values the fluorescence in wells without test compound (1% DMSO, negative control) is set as 100% enzymatic activity and the fluorescence in wells with 2 μM TSA (positive control) are set at 0% enzymatic activity (background fluorescence substracted).
|
|
| In vivo |
Oral resminostat at 600 mg QD continuously d1−5 in a 14 day cycle is well-tolerated. This compound shows a favourable PK profile, with high bioavailability and low inter-pt variability. The apparent t 1/2 of this compound ranged from 2.7 to 4.4 hours. The modulation of plasma biomarkers further indicates drug activity.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04955340 | Completed | Cutaneous T Cell Lymphoma|Mycosis Fungoides|Sezary Syndrome |
4SC AG |
October 12 2021 | Phase 1 |
| NCT01277406 | Completed | Advanced Colorectal Carcinoma |
4SC AG |
January 2011 | Phase 1|Phase 2 |
| NCT01037478 | Completed | Hodgkin''s Lymphoma |
4SC AG |
December 2009 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































